Answer:
Cellular respiration is spontaneous and exergonic. And the energy released during this process is used primarily for the formation of new chemical bonds (ATP)
Step-by-step explanation:
In the catabolic reactions complex substances are transformed into simpler ones. When the molecules are degraded, they release the energy contained in the chemical bonds. This is the type of energy that is produced in cellular respiration.
Cellular respiration is an exergonic reaction, where part of the energy contained in the food molecules is used by the cell to synthesize ATP, which is a small molecule that powers reactions in the cell. It is said that part of the energy is detected for synthesis, because much of the energy is dissipated as heat.
In chemistry, a spontaneous process is one that occurs without the external energy supply. And generally spontaneity is analyzed by Gibbs energy (G), which is a measure of the amount of usable energy. When ΔG <0 process is exergonic and will occur spontaneously directly to form more products.
When ΔG> 0 the process is endergonic and is not spontaneous in the direct sense. On the contrary, it will occur spontaneously in the opposite direction to produce more reagents.
When ΔG = 0, the system is in equilibrium and the concentrations of products and reagents will remain constant.
The general reaction of the analyzed process can write as follows:
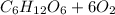 ⇒
⇒
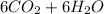
ΔG=-686
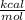
As you can see, G is less than zero. It was also mentioned that cellular respiration is an exergonic reaction. Then it can be said that it is a spontaneous reaction.
In summary, cellular respiration is spontaneous and exergonic. And the energy released during this process is used primarily for the formation of new chemical bonds (ATP)