Answer:
A) & B)
Step-by-step explanation:
First, the numbers 12 and 13 represent the atomic mass number of the atoms.
- So, A) is true: thus Carbon12 and Carbon 13 have different mass numbers.
The mass number is equal to the total number of protons and neutrons. Consider that any element has the same number of protons, regardless of the number of neutrons. The number of protons in Carbon is 6.
The amount of neutrons can be calculated by: mass number minus number of protons.
For Carbon 12:
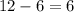
For Carbon 13:
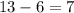
- B) is true, we just proved they have different amounts of neutrons.
In order for the charge of the atom to be neutral, the amount of electrons must be equal to the number of protons (as they have opposite charges). And we now know that the number of protons in Carbon12 and Carbon13 are always the same
- C) is false, the number of electrons is the same in both atoms
The atomic charges in both are neutral, due to the fact that they have the same amount of protons and electrons in both cases. Is only the neutrons (thus the mass numbers)that change
- D) is false, they have equal atomic charges