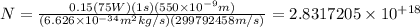Answer:
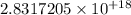 photons
photons
Step-by-step explanation:
Infrared radiation also consists of photons, but we will assume they mean visible photons.
The energy of a photon is given by the formula
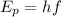 , where f is the frequency of the photon and
, where f is the frequency of the photon and
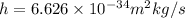 is Planck's constant.
is Planck's constant.
The relationship between frequency and wavelength is given by
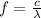 , where c=299792458m/s is the speed of light.
, where c=299792458m/s is the speed of light.
The energy of N photons will be then given by:
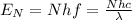
Which can be written as:
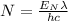
We need to know how much energy do we have. The power we have relates to the energy by the equation P=E/t, but of this energy we only get 15% as visible, so the energy related to the visible photons will be 0.15E.
Putting all together:
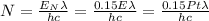
We then substitute our values, considering only 1s: