Answer:
Wavelength,
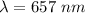
Step-by-step explanation:
The energy of the electron in a hydrogen atom can be calculated from the Bohr formula as :
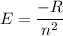 .............(1)
.............(1)
Where
R is the Rydberg constant
n is the number of orbit
We need to find the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with to an orbital with n₁ = 2 to an orbital with n₂ = 3.
Equation (1) can be re framed as :
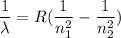
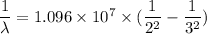
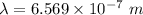
or
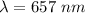
So, the the wavelength of the line in the absorption line spectrum is 657 nm. Hence, this is the required solution.