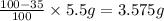Answer:
The correct answer is option (1).
Step-by-step explanation:
Mass of the sample = 5.5 g
Percentage of carbon in sample = 35 %
Percentage of oxygen in sample = 100% - 35%
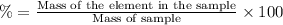
Mass of carbon in a sample: (35%) of 5.5 g
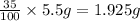
Mass of oxygen in a sample: (100%-35%) of 5.5 g