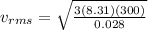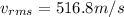Answer:
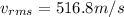
Step-by-step explanation:
RMS speed is root mean square speed of ideal gas molecules
It is defined as the average value of the speed of gas molecules which is given as
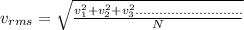
this above expression can be also calculated by the kinetic energy of the gas molecules
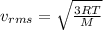
here we know that
T = temperature of gas
M = molar mass
now for nitrogen molecule at room temperature the RMS speed is given as