Answer : The correct option is, (B) 0.82 M
Explanation : Given,
The dissociation constant for formic acid =
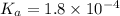
Concentration of formic acid (weak acid)= 0.72 M
pH = 3.80
First we have to calculate the value of
 .
.
The expression used for the calculation of
 is,
is,
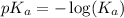
Now put the value of
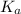 in this expression, we get:
in this expression, we get:
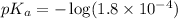
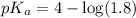
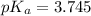
Now we have to calculate the concentration of sodium formate (conjugate base or salt).
Using Henderson Hesselbach equation :
![pH=pK_a+\log ([Salt])/([Acid])](https://img.qammunity.org/2020/formulas/chemistry/college/6wyuhr9b7n0qwlgrnwgg688yylfbvv3wby.png)
Now put all the given values in this expression, we get:
![3.80=3.745+\log (([Salt])/(0.72))](https://img.qammunity.org/2020/formulas/chemistry/college/2k3mpvn11lho2jwb2cd570eebmjg6xid9j.png)
![[Salt]=0.82M](https://img.qammunity.org/2020/formulas/chemistry/college/ryw6bdffmg7wvmzfx6ogkk3e9ip242vs1c.png)
Therefore, the concentration of sodium formate is 0.82 M.