Answer:
A. The volume will increase.
Step-by-step explanation:
As per Avagadro’s law
At constant temperature and pressure, the volume occupied by a gas is directly proportional to the number of moles of the gas.
Directly proportional means when one increases the other one too will increase. That is, Equal volume of different gases will have the same number of moles at constant temperature and pressure.
1 mole of Hydrogen gas at T = 273K and P = 1 atm occupies a volume of 22.4L
1 mole of Argon gas at T = 273K and P = 1 atm occupies a volume of 22.4L
This is given by the expression
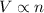 (at constant temperature and pressure)
(at constant temperature and pressure)
Introducing a constant we have
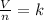
Where V is the volume, n is the number of moles and k represents a constant.
When there is a change in volume or number of moles we make use of the expression
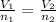
As volume increases the number of moles occupies too will increase.