Answer:
The reverse reaction is more favourable than the forward reaction.
Step-by-step explanation:
The value of equilibrium constant in this case is
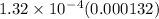 . A high value of equilibrium constant indicates that the forward reaction is more favourable.
. A high value of equilibrium constant indicates that the forward reaction is more favourable.
Thus the resultant of the reaction will have higher concentration of products. When the value of equilibrium constant is less the reverse reaction will be more favoured than the forward reaction.
Thus the resultant of the reaction will have more reactants than products. Here equilibrium constant has a very low value which favours reverse reaction.