Answer:
The correct answer is option C.
Step-by-step explanation:
Heat of formation is defined as change in enthalpy during the formation of 1 mole of compound from its constituent elements in its standard states.it is denoted by symbol
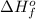 . Its units are Kilo joules per mole.
. Its units are Kilo joules per mole.
- If
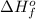 <0, the change in enthalpy is negative then it means that energy is released during the formation of 1 mole of substance.
<0, the change in enthalpy is negative then it means that energy is released during the formation of 1 mole of substance. - If
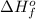 >0 , the change in enthalpy is positive then it means that energy is absorbed during the formation of 1 mole of substance.
>0 , the change in enthalpy is positive then it means that energy is absorbed during the formation of 1 mole of substance.
For example:
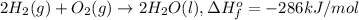
286 kilo Joules of energy given out when 1 mole of liquid water is formed.