Step-by-step explanation:
A precipitate is defined as an insoluble solid which is formed due to chemical combination of two aqueous solutions.
For example,
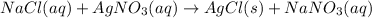
Here, AgCl is the precipitate which is formed.
When a precipitate is present in a solution then either it settles at the bottom or remain suspended into the solution leading to the formation of a cloudy solution.
This means that a cloudy solution also indicates that a solid is being formed.
Similarly, a gel is also a solid like substance which is able to retain its shape. Therefore, formation of a gel also indicates a solid is being formed.
Whereas formation of bubbles indicate that a gas is being formed. And, change in color or change in temperature of the solution does not indicate any change in state of the substance.
Thus, we can conclude that out of the given options three of the following indicate that a solid is being formed.
- formation of a precipitate.
- formation of a cloudy solution.