Answer:
The mass of water = 54 g
Mass of sodium carbonate = 6 g
Step-by-step explanation:
- To calculate the mass needed when given the volume of water and percentage mass we can use the formula;
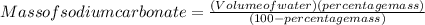
In this case;
Percentage mass = 10 %
Total mass of solution = 60 g
Assuming the volume of water = y cm³, the mass of water is y g
Then, the mass of sodium carbonate(solute)= 60-y
Hence;
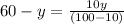
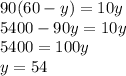
Therefore;
The mass of water = 54 g
Mass of sodium carbonate = 6 g