Answer:
the relative atomic mass of 25-Mg is 25.05539
Solution and Explanation:
- Isotopes are atoms that belong to the same element having the same number of protons, however they have a different number of neutrons making them differ in their mass number.
- Relative atomic number is calculated by getting the sum of product of the mass of atoms and their relative abundances then dividing by 100.
In this case, magnesium has three isotopes with different masses and relative abundances. We need to calculate the atomic mass of 25-Mg
Step 1: Calculating relative abundances of 26-Mg and 25-Mg
24 Mg - atomic mass of 23.98504 and relative abundance of 78.99%
26 Mg - atomic mass of 25.98259 and relative abundance of y %
25 Mg - atomic mass of x and relative abundance of 0.9083y%
But, the sum of relative abundances add up to 100%
Therefore;
y + 0.9083y + 78.99 = 1000
1.9083y = 21.01
y = 11.00980
Thus, the relative abundances of 26-Mg and 25-Mg are 11.00980 and 10.0002 respectively.
Step 2: Calculating the atomic mass of 25-Mg
R.A.M of Mg is 24.312
Assuming the atomic mass of 25-Mg is x
Then;
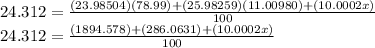
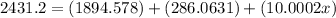
Solving for x
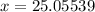
Therefore the relative atomic mass of 25-Mg is 25.05539