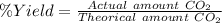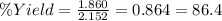Answer:
86.4%
Step-by-step explanation:
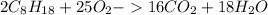
MM (C₈H₁₈) = 12*8+1*18 = 114
MM (CO₂) = 12 + 16*2 = 44
moles of C₈H₁₈ = 30.65 / 114 = 0.269
moles of CO₂ = 81.75 / 44 = 1.860
The % Yield is the amount of a product formed divided by the theorical amount formed if the limiting reactant reacts 100%.
The amount formed of CO₂ is 1.860 moles
The theorical amount formed is obtained with the rule of three and the stoichiometric coefficients.
2 moles of C₈H₁₈ produces 16 moles of CO₂
0.269 moles of C₈H₁₈ produces x moles of CO₂
x = 0.269 * 16 / 2 = 2.152 moles