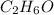Answer:
Step-by-step explanation:
For a compound as:
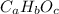
Let say your sample weighs 100 g, then you have
52 g of C
35 g of O
13 g of H
Now you need to calculate the moles in the sample:
moles of C = 52 / 12 = 4.3333
moles of O = 35 / 16 = 2.1875
moles of H = 13 / 1 = 13
Now you divide the moles by the least to have a proportion (2.1875):
moles of C = 4.3333 / 2.1875 = 2
moles of O = 2.1875 / 2.1875 = 1
moles of H = 13 / 2.1875 = 6
So the molecule proportion of atoms is C:H:O = 2:6:1
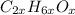
Now to find x you have to calculate the MM:
MM
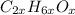 = 12 * 2x + 1 * 6x + 16 * x = 46x
= 12 * 2x + 1 * 6x + 16 * x = 46x
For x = 1 => MM = 46(1) = 46
For x = 2 => MM = 46(2) = 92
Since the MM is between 30 and 80, x = 1