Answer:
E = (h2 / 8mL2) (n1² /4 + n2² + n3²) ground state (1 0 0)
Step-by-step explanation:
The solution of the Schrödinger equation for a potential box with infinite walls is a relatively simple state described in one dimension by the equation
E = (h² / 8mL²) n²
Where h is the Planck constant 6.63 10 -34 J s, m the mass of the particle, L the length of the box, n is an integer that in quantum mechanics is called quantum number
Let's apply this equation to our case
X axis
E = (h² / 8mLₓ²) n1²
Lx = 2L
E1 = (h² / 8m (2L)²) n1²
E1 = (h² / 8mL²) ¼ n1²
Y Axis
E2 = (h² / 8m
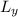 ²) n2²
²) n2²
Ly = L
E2 = (h2 / 8mL²) n2²
Z axis
E3 = (h2 / 8m
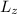 ²) n3²
²) n3²
Lz = L
E3 = (h² / 8mL²) n3²
As energy is a scalar and is additive
E = E1 + E2 + E3
E = (h² / 8mL²)) ¼ n1² + (h² / 8mL²) n2² + (h² / 8mL²)) n3²
Let's simplify
E = (h2 / 8mL2) (n1² /4 + n2² + n3²)
Let's call Eo = (h² / 8mL²) to simplify the equation a bit
E = Eo (1/4 n1²+ n2² + n3²)
The base state is the lowest energy state
If we inspect the equation above the state of lowest energy acure for
n1 = 1 n2 = 0 n3 = 0
E = (h² / 8mL²) (¼ + 0 +0)
E = ¼ Eo
The first excited states are
n1 n2 n3 E
0 0 1 Eo
0 1 0 Eo
1 0 1 E0 (1+ ¼) = 5/4 Eo
1 1 0 5/4 Eo
1 1 1 Eo (¼ + 1 + 1) = 9/4 Eo