Answer: The reaction is not at equilibrium and will proceed to make more products to reach equilibrium.
Explanation:
Equilibrium constant is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios. It is expressed as

K is the constant of a certain reaction when it is in equilibrium, while Q is the reaction quotient of activities of products and reactants at any stage other than equilibrium of a reaction.
For the given chemical reaction:
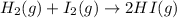
The expression for
 is written as:
is written as:
![Q=([HI]^2)/([H_2]^1[I_2]^1)](https://img.qammunity.org/2020/formulas/chemistry/college/npjx7hshryewuwq6pjrztr5kj93o2m9pi3.png)
![Q=([0.0890]^2)/([0.215]^1[0.498]^1)](https://img.qammunity.org/2020/formulas/chemistry/college/x9x440k9jsi083hq3m365roz0wepgvaz1l.png)
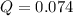
Given :
 = 54.8
= 54.8
Thus as
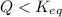 , the reaction will shift towards the right i.e. towards the product side.
, the reaction will shift towards the right i.e. towards the product side.