Answer:
The specific heat is 3.47222 J/kg°C.
Step-by-step explanation:
Given that,
Temperature = 13°C
Temperature = 37°C
Mass = 60 Kg
Energy = 5000 J
We need to calculate the specific heat
Using formula of energy
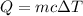
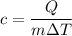
Put the value into the formula
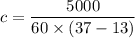
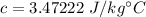
Hence, The specific heat is 3.47222 J/kg°C.