Answer:
Step-by-step explanation:
When absorbing light, we use the UV Vis sprectrophotometre. When using this object we measure the peak at which our sample will absorb which in this case is 800nm. Then for calculating anything regarding this experiment, we use the following eq
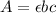
where A is absorbance, e is the coefficient of molar absorbance, b the lenght of the box (L) and c the concentration of the sample. By using this equation, and having A, e and c we can obtaing L.