Answer:
A)
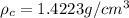
B) 66.6%
Step-by-step explanation:
A) expression for crystallanity is given as
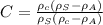 ........1
........1
where, \rho_c = density of crystalline, \rho_a = density of amorphous material,
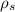 = density of specimen
= density of specimen
C = 0.743 [given]
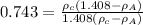
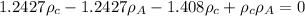
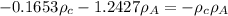 ........2
........2
SUBSTITUTE
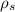 value of second specimen in 1 equation we get
value of second specimen in 1 equation we get
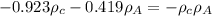 .......3
.......3
ON COMPARING 2 AND 3rd equation we get
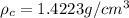
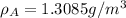
b)
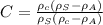
substitute value to ger crystallanity
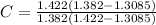
C = 66.6 %