Step-by-step explanation:
The ionic reaction of the copper and the silver ions is shown below:
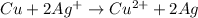
Copper gives up 2 electrons and transfers to 2 silver ions which gives solid silver (reduction) and copper (II) ions.
The blue color of the solution is due to the formation of copper (II) ions. The electrons in the copper ion shows d-d transition which absorbs light in the visible region and shows blue color.