Answer: Option (b) is the correct answer.
Step-by-step explanation:
Average kinetic energy is defined as half of mass of gas times the square of root mean square velocity.
Mathematically, Average K.E =
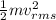
Also, Kinetic energy =
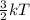
So, when there is increase in temperature then there will be increase in movement of the molecules. As a result, kinetic energy will also increase. Since, kinetic energy is proportional to temperature.
Therefore, with decrease in temperature there will be decrease in average kinetic energy of the molecules.
Thus, we can conclude that the average kinetic energy of the molecules of a warm substance also decreases when the temperature of the substance is slightly decreased.