Answer:
38,456.4 grams of urea is produced per minute by this reaction.
Step-by-step explanation:
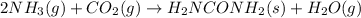
Ammonia gas at 223°C and 90 atm flows into a reactor at a rate of 580 L/min.
Volume of ammonia = V = 580 L/min
Temperature of the gas = T = 223°C = 496 K
Pressure of the ammonia gas = P = 90 atm
Moles of ammonia per minute = n

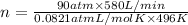
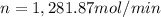
Carbon dioxide at 223°C and 48 atm flows into the reactor at a rate of 600 L/min.
Volume of gas= V = 600 L/min
Temperature of the gas = T = 223°C = 496 K
Pressure of the ammonia gas = P = 48 atm
Moles of ammonia per minute = n

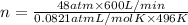
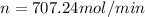
Moles of ammonia gas per minute = 1,281.87 mol
Moles of carbon dioxide per minute = 707.24 mol
According to reaction 2 mol of ammonia gas reacts with 1 mol of carbon dioxide.
Then 1,281.87 moles of ammonia will react with:
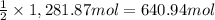 of carbon dioxide.
of carbon dioxide.
Carbon dioxide is in excess amount. Amount of urea will depend upon amount of ammonia.
According to reaction 2 mol of ammonia gas gives 1 mol of urea.
Then 1,281.87 moles of ammonia will give:
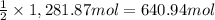 of urea.
of urea.
Mass of 640.94 moles of urea : 640.94 mol × 60 g/mol=38,456.4 g
38,456.4 grams of urea is produced per minute by this reaction.