Answer:
The actual yield of iron in moles is 34.5%
Step-by-step explanation:
The balanced chemical equation is
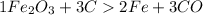
The conversions are moles
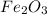 to moles Fe and moles Fe to mass Fe
to moles Fe and moles Fe to mass Fe
Mole ratio of
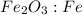 is 1 : 2
is 1 : 2
So using mole ratio we see
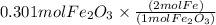
=0.601 mol Fe is produced.
By multiplying with molar mass of Fe we can convert moles Fe to mass Fe.
That is
Mass Fe = moles Fe × molar mass Fe
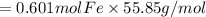
=33.566 g Fe produced.
(Theoretical yield)
11.6 g given is the actual yield.
% yield=(actual yield )/(Theoretical yield )×100%
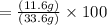
= 34.5% is the Answer