Answer:
Protons:
- Positively charged particle
- The number of these is the atomic number
- All atoms of a given element have the same number of these
Neutrons:
- Isotopes of a given element differ in the number of these
- The mass number is the number of these added to the number of protons
Step-by-step explanation:
Protons (positively charged), neutrons (neutral) and electrons (negatively charged) are smaller than an atom and they are the main subatomic particles. The nucleus of an atom is composed of protons and neutrons, and the electrons are in the periphery at unknown pathways.
The Atomic number (Z) indicates the number of protons (
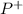 ) in the nucleus. Every atom of an element have the same atomic number, thus the same number of protons.
) in the nucleus. Every atom of an element have the same atomic number, thus the same number of protons.
The mass number (A) is the sum of the number of protons (
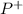 ) and neutrons (N) that are present in the nucleus: A= Z + N
) and neutrons (N) that are present in the nucleus: A= Z + N
Isotopes are atoms of the same element which nucleus have the same atomic number (Z), and different mass number (A), it means the same number of protons (
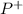 ) and a different number of neutrons (N). For example, the oxygen in its natural state is a mixture of isotopes:
) and a different number of neutrons (N). For example, the oxygen in its natural state is a mixture of isotopes:
99.8% atoms with A= 16, Z=8, and N=8
0.037% atoms with A=17, Z=8, and N=9
0.204% atoms with A=18, Z=8, and N=10