The seashell is 28747.96 years old.
Why?
If we need to calculate how old is something, we can use the equation for radioactive decay rate. It's possible using the C-14 half-life (5740 years) as reference. Remember, C-14 is used because all the living things take up the element from the atmosphere, when an organism dies, the amount of carbon starts to decay (very slowly).
Decay rate half life:
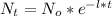
Where,
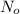 is the initial number of atoms (undecayed)
is the initial number of atoms (undecayed)
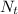 is the the number of atoms at time (undecayed)
is the the number of atoms at time (undecayed)
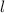 is the decay rate
is the decay rate
Now, isolating the decay rate of the formula, we have:
{
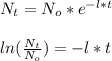
Also, we can get the value of the decay rate (half life), using the following formula:
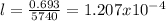
Then, using the given information, we have:
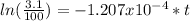
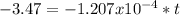
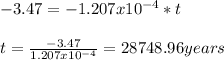
Hence, the seashell is 28748.96 years old.
Have a nice day!