Answer:
For A: The number of arsenic atoms are
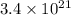
For B: The percent composition of mercury, thallium and chromium in their complexes are 61.76 %, 62.2 % and 29.51 % respectively.
Step-by-step explanation:
To calculate the number of moles, we use the equation:

Given mass of dimercaprol = 696 mg = 0.696 g (Conversion factor: 1 g = 1000 mg)
Molar mass of dimercaprol = 124.21 g/mol
Putting values in above equation, we get:
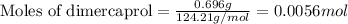
According to mole concept:
1 mole of a compound contains
 number of molecules.
number of molecules.
So, 0.0056 moles of dimercaprol will contain
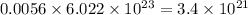 number of molecules.
number of molecules.
As, 1 molecule of dimercaprol binds with 1 atom of Arsenic
So,
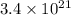 number of dimercaprol molecules will bind with =
number of dimercaprol molecules will bind with =
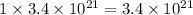 number of arsenic atoms
number of arsenic atoms
Hence, the number of arsenic atoms are
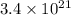
We know that:
Molar mass of dimercaprol = 124.21 g/mol
Molar mass of mercury = 200.59 g/mol
Molar mass of thallium = 204.38 g/mol
Molar mass of chromium = 51.99 g/mol
Also, 1 molecule of dimercaprol binds with 1 metal atom.
To calculate the percentage composition of metal in a complex, we use the equation:
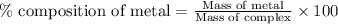 ......(1)
......(1)
Mass of Hg-complex = (200.59 + 124.21) = 324.8 g
Mass of mercury = 200.59 g
Putting values in equation 1, we get:
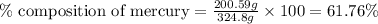
Mass of Tl-complex = (204.38 + 124.21) = 328.59 g
Mass of thallium = 204.38 g
Putting values in equation 1, we get:
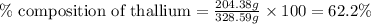
Mass of Cr-complex = (51.99 + 124.21) = 176.2 g
Mass of chromium = 51.99 g
Putting values in equation 1, we get:
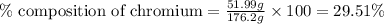
Hence, the percent composition of mercury, thallium and chromium in their complexes are 61.76 %, 62.2 % and 29.51 % respectively.