Answer:
Step-by-step explanation:
Hello, in this case, the lead is catching heat and the water losing it, that's why the heat relation ship is (D is for Δ):
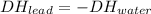
Now, by stating the heat capacity definition:
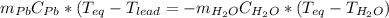
Solving for the equilibrium temperature:
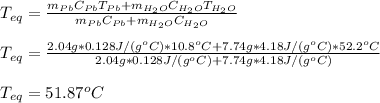
Which is very close to the water's temperature since the lead's both mass and head capacity are lower than those for water.
Best regards.