Answer : The correct option is, 22.4 L
Explanation :
STP : STP stands for standard temperature and pressure of the gas.
As we know that at STP,
The temperature of gas is,
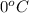 or
or
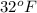 or 298 K.
or 298 K.
The pressure of gas is,
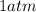 or
or
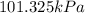
The volume of gas is, 22.4 L
That means, 1 mole of substance occupies 22.4 liter volume of gas at STP.
Hence, the correct option is, 22.4 L