Answer: Option (b) is the correct answer.
Step-by-step explanation:
Number of moles given are 0.5 mol and molar mass of tyrosine (
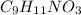 ) is 181.19 g/mol.
) is 181.19 g/mol.
Therefore, calculate the mass of given molecules follows.
No. of moles of tyrosine =
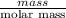
0.5 mol =
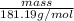
mass = 90.595 g
Therefore, we can conclude that mass of 0.5 mole of tyrosine (
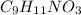 ) is 90.5 g.
) is 90.5 g.