Answer:
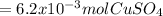
Step-by-step explanation:
Hello,
By developing the following stoichiometric relationship, the required amount could be found as follows:
- Moles of
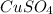 :
:
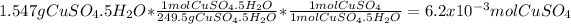
- Grams of
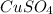
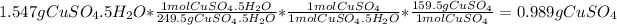
- Moles of water:
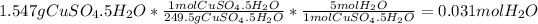
Finally, one could see that the mass of the anhydrous compound is less than the pentahydrated compound since it is waterless.
Best regards.