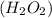Answer: The correct answer is 1 mole of peroxide
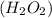
Step-by-step explanation:
According to mole concept:
1 mole of a substance contains
 number of atoms.
number of atoms.
For the given options:
Option 1: 1 mole of hydrogen peroxide
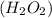
1 mole of hydrogen peroxide contains 2 moles of hydrogen atom and 2 moles of oxygen atoms.
This means that 1 mole of hydrogen peroxide contains
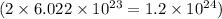 number of oxygen atoms.
number of oxygen atoms.
Option 2: 1 mole of water
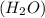
1 mole of water contains 2 moles of hydrogen atom and 1 moles of oxygen atoms.
This means that 1 mole of water contains
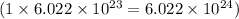 number of oxygen atoms.
number of oxygen atoms.
Option 3: 2 moles of ammonia
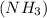
1 mole of ammonia contains 1 moles of nitrogen atom and 3 moles of hydrogen atoms.
This compound do not contain any oxygen atom.
Option 4: 2 moles of hydrogen gas
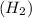
1 mole of hydrogen gas contains 2 moles of hydrogen atoms.
This compound do not contain any oxygen atom.
Hence, the correct answer is 1 mole of peroxide