Answer:
liquid state of lead T = 547ºC
Step-by-step explanation:
The temperature change of the lead bullet can be found by the calorimetry equation
Q = m
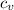 ΔT
ΔT
Where heat is the energy transferred to lead during the crash, we can find average work
W = F x
With the crash it is very short we can use the average speed of the projectile during the crash
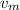 = (
= (
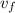 + v₀) / 2 = (600 + 300) / 2
+ v₀) / 2 = (600 + 300) / 2
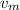 = 450 m / s
= 450 m / s
This speed of the projectile inside the wall is very inaccurate, but it is better than using any of the two speeds given (initial and final)
W = Δp vm
W = (m
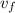 -m v₀) vm
-m v₀) vm
W = m (
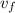 -v₀) vm
-v₀) vm
W = 30 10⁻³ (300-600) 450
W = 4050 J
As we have a shock let's use the momentum
I = F t = ΔP
F = Δp / t
Let's replace
W = (Dp / t) x
With the crash it is very short we can use the average speed of the projectile during the crash
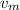 = (
= (
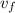 + v₀) / 2 = (600 + 300) / 2
+ v₀) / 2 = (600 + 300) / 2
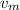 = 450 m / s
= 450 m / s
This speed of the projectile inside the wall is very inaccurate, but it is better than using any of the two speeds given (initial and final)
W = Δp vm
W = (m
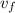 -m v₀) vm
-m v₀) vm
W = m (
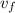 -v₀) vm
-v₀) vm
W = 30 10⁻³ (300-600) 450
W = 4050 J
They tell us that 50% of the heat is absorbed by lead
Q = 50% W
Q = 2025 J
Let's calculate the temperature , the specific heat of lead is
cv = 128 J/kg ºC
Q = m
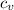 Δt
Δt
ΔT = Q / m
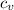
ΔT = 2025 / 3010⁻³ 128
 = 547C
= 547C
If we review the periodic table, the melting point of lead is 327.3ºC and the boiling point is 1726ºC, since it has a higher temperature than the lead must be in a liquid state