Answer:
The pH of the HCl solution is 3.9
Step-by-step explanation:
Knowing the molar mass of HCl (36.5g/mol) it is possible to calculate the number of moles of HCl in the solution,
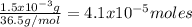 of HCl
of HCl
HCl is a strong acid and when added to water will form H+ and Cl-, thus the moles of HCl are equal to moles of H+ in 354 mL of water
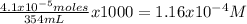
The concentration of protons in the solutions is
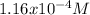 .
.
The expression used to calculate the pH is
![pH = -Log ([H^(+)]) = -Log (1.16x10^(-4) M) = 3.9](https://img.qammunity.org/2020/formulas/chemistry/college/bufewh00b0q6yfq32wb9p54ovudmhb7k1f.png)
Hence, the pH of the HCl solution is 3.9 and Pepsi has a pH of 3.4 approximately, so they have almost the same pH.