Step-by-step explanation:
Calcium oxide absorbed moisture (water vapors ) from the air and forms calcium hydroxide as a product which why it is stored in dry place in a chemistry laboratory.
The chemical reaction between calcium oxide and moisture is given as:
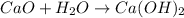
According to stoichiometry, 1 mole of calcium oxide reacts with 1 mole of water vapors to give 1 mole of calcium hydroxide.