Answer:
The heat of reaction is 4938 kJ/mole
Step-by-step explanation:
It is possible to calculate the heat of a reaction using:
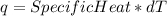
For the reaction:
C₁₂H₂₂O₁₁ (s) + 12 O₂ (g) → 12 CO₂ (g) + 11 H₂O (l)
Specific heat: 7,50kJ/°C
dT = 20,2°C
Thus, q = 151,5 kJ
The moles of sucrose are: 10,5g /342,2965 g/mol = 0,030675 moles
The heat of reacton is:
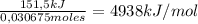
I hope it helps!