ANSWER:
110ML of 35% acid solution must be mixed with 10ML of 95% solution to obtain 120ML of 40% solution.
SOLUTION:
First, set up table. fill in the unknowns with variables x and y. The table is attached below.
From the table shown below, we can easily set up the two equations.
Sum of values of two acids = Value of mixture
0.35x + 0.95y = 48
For convenience, we will multiply the entire equation by 100,
35 x + 95y = 4800 ------ (1)
Now, Sum of amounts of each acid = Amount of mixture
x + y = 120 --------- (2)
Multiply eqn 2 with 35 for easy calculation and derive the equation into one variable.
35 = 35x + 35y = 4200
Subtracting equation (2) from (1), we get
0 + 60y = 600
Thus, 60y = 600
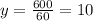
Substituting y = 10 in (2),
35x + 35(10) = 4200
35x + 350 = 4200
35x = 4200 - 350
35x = 3850
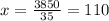
So, we have x = 110 and y = 10
We can conclude that 110ML of 35% acid solution must be mixed with 10ML of 95% solution to obtain 120 ML of 40% solution.