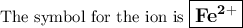Answer:
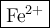
Step-by-step explanation:
1. Number of electrons
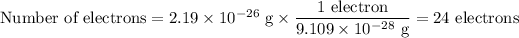
2. Number of protons
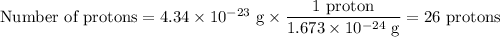
3. Identify the ion
An atom with 26 protons is iron, Fe.
A neutral atom of iron would have 26 electrons.
The ion has only 24 electrons, so it has lost two. The ion must have a charge of +2.