Answer:
0.3096g of glucose per minute
Step-by-step explanation:
Given parameters:
Number of moles of O₂ needed per minute = 1.03 x 10⁻²mol
Unknown:
Mass of glucose the body consumes per minute =?
Solution:
The given balanced equation of the reaction is shown below:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O
We have been given the exact amount of all the oxygen gas required for the cellular respiration process per minute. Using this value, we can know how the reaction will proceed successfully.
From the balanced equation:
6 moles of O₂ reacted with 1 mole of C₆H₁₂O₆
therefore 1.03 x 10⁻² of O₂ will react with
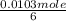 ;
;
⇒ 0.00172mole of glucose will react per minute.
To find the mass in grams of the glucose that will react, we use the expression below"
Mass of glucose = Number of moles of glucose x molar mass of glucose
Molar mass of C₆H₁₂O₆ = (12 x 6) + (12 x 1) + (16 x 6) = 180g/mol
Mass of glucose = 0.00172mole x 180g/mole = 0.3096g of glucose per minute