Answer:
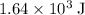 .
.
Step-by-step explanation:
Assume that his calorimeter is sufficiently effective, such that no heat had escaped to the surroundings. Heat from this solution would be absorbed by either
- the solution, or
- the coffee cup.
Temperature change:
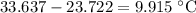 .
.
Heat absorbed by the solution:
Only the specific heat capacity (per unit mass) of the solution is given. Both the mass of the solution and the temperature change will be required for determining the energy change. Start by finding the mass of the solution.
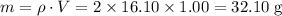 .
.
Calculate the amount of heat absorbed from the specific heat:
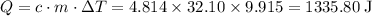 .
.
Heat absorbed by the coffee cup:
The heat capacity of the coffee cup is given. Only the temperature change will be required for finding the amount of heat absorbed.
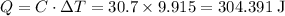 .
.
Heat that this reaction produces
Find the sum of the two parts of heat. Round to three significant figures as in the heat capacity of the coffee cup and the density of the solution.
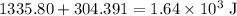 .
.