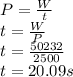Answer:
t=20s
Step-by-step explanation:
To solve this problem we must apply the first law of thermodynamics, which indicates that the energy that enters a system is the same that must come out, resulting in the following equation
For this problem we will assume that the water is in a liquid state, since it is a domestic refrigerator
q=m.cp.(T2-T1)
q=heat
m=mass of water =600g=0.6Kg
cp=
specific heat of water=4186J/kgK
T2=temperature in state 2=20°C
T1=temperature in state 1=0°C
solving:
q=(0.6)(4186)(20-0)=50232J
A refrigerator is a device that allows heat to be removed to an enclosure (Qin), by means of the input of an electrical energy (W) and the heat output (Qout), the coefficient of performance COP, allows to know the ratio between the heat removed ( Qin) and the added electrical power (W), the equation for the COP is
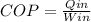
To solve this exercise we must know the value of the heat removed to the water (Qin)
solving for Qin
Qin=(COP)(Win)
Qin=(5)(500W)=2500W
finally we remember that the definition of power is the ratio of work over time
w=work
p=power=500w