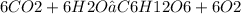Question :-
Is 6CO² + 6H²O → C⁶H¹²O⁶ + O² a balanced chemical equation
Answer :-
No it's not a balanced equation .
We can se that total number of Oxygen atom on left side is 18 which is more than the number of Oxygen on the right which is 7
we know that matter can't be created nor be destroyed in a chemical reaction so then given equation doesn't follow this rule . we can say that this equation is incomplete
The Balanced Equation :-