The Law of Multiple Proportions states that if 2 elements form more than one compound between them, if the mass of one of the elements is fixed, the masses of the other element will be in a ratio of small whole numbers.
In this case, the two elements are Fe and Cl, and the 2 compounds are X and Y. Let us fix the mass of Fe in 1.00 g and analyze what happens with the masses of Cl.
For X:
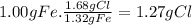
For Y:
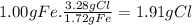
The ratio mCl in X/mCl in Y is 1.27g/1.91g = 0.664 ≅ 2/3. This is a ratio of small whole numbers, which proves the Law of Multiple Proportions.