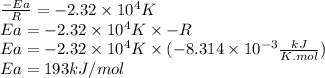Answer:
The activation energy for the gas phase isomerization of cis-cyanostyrene is 193 kJ/mol.
Step-by-step explanation:
The relation between the rate constant (k) and the absolute temperature (T) is given by the Arrhenius equation:
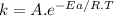
where,
A is a preexponential factor
Ea is the activation energy
R is the ideal gas constant
T is the absolute temperature
This expression can be re-written like:
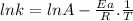
The plot of ln k against 1/T gives a linear plot where ln A is the intercept and -Ea/R is the slope.
Since we know the slope is -2.32 × 10⁴ K, we can find Ea: