Step-by-step explanation:
Formation of formate buffer will be as follows.
Formate buffer = HCOOH + HCOONa
Molar mass of HCOOH
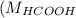 = 46 g/mol
= 46 g/mol
Molar mass of HCOONa
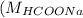 = 68 g/mol
= 68 g/mol
 of HCOOH = 3.75
of HCOOH = 3.75
As,
![[HCOO^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/snqk4v747j27eepk3wer6cvf7wx55y3j4e.png) = [HCOOH] + [HCOONa]
= [HCOOH] + [HCOONa]
= [HCOONa] (here, dissociation of HCOOH is negligible)
Its preparation will be as follows.
750 ml of 0.25 M sodium formate buffer
Since, there are many combinations of salt + acid and among those possibilities one of them is as follows.
HCOONa = 0.25 M in 400 ml solution
HCOOH = x molat in 350 ml solution
Therefore as, pH =
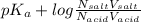
4 = 3.75 +
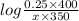
x = 0.16 M
Therefore, molarity of the formic acid acid is 0.16 M.
Now, calculate the weight of sodium formate as follows.
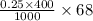
= 6.8 g
And, number of milliliters of formic acid required is 350 ml.