Answer:
Mass of KNO3 in the original mix is 146.954 g
Step-by-step explanation:
mass of
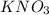 in original 254.5 mixture.
in original 254.5 mixture.
moles of
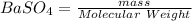
moles of
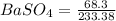
= 0.2926 mol of BaSO4
Therefore,
0.2926 mol of BaCl2,
mass of
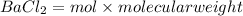
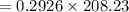
= 60.92 g
the AgCl moles
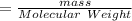
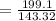
= 1.3891 mol of AgCl
note that, the Cl- derive from both,
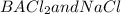
so
mole of Cl- f NaCl
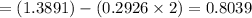 mol of Cl-
mol of Cl-
mol of NaCl = 0.8039 moles
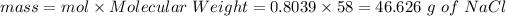
then
KNO3 mass = 254.5 - 60.92-46.626 = 146.954 g of KNO_3
Mass of KNO3 in the original mix is 146.954 g