Answer:
The concentration of the acid is 117.92 ± 0.03 mM.
Step-by-step explanation:
In this problem, the concentration of NaOH and the volume used is given. The volume pipetted from the acid is also given. The concentration of the acid is given by:
CNaOH * VNaOH = Cacid * Vacid
158.32 * (18.76-0.14) = C acid * 25
C acid = 117.92 mM
The propagation of error for this problem, which is a multiplication is the concentration of the acid multiplied by the error divided by its respective value as shown in the equation below
error = 117.92 *
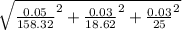
The answer is 0.03 mM of uncertainty.