Answer: Option (b) is the correct answer.
Step-by-step explanation:
When a compound contains a strong bond between its combining atoms or molecules then the compound will have a high boiling point.
An ionic bond is a strong bond because it contains strong force of attraction between two oppositely charged atoms.
For example, LiF is an ionic compound in which lithium is
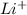 ion and fluorine is
ion and fluorine is
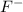 ion.
ion.
Therefore, LiF compound will have strong force of attraction.
On the other hand, covalent compounds have weak bond due to sharing of electrons. Hence, they have low boiling point.
For example,
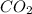 ,
,
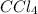 , and
, and
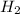 etc are covalent compounds.
etc are covalent compounds.
Thus, we can conclude that LiF substance has the highest boiling point.