Answer: The theoretical yield of sodium sulfate is 1.30 grams.
Step-by-step explanation:
To calculate the number of moles, we use the equation:
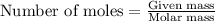 .....(1)
.....(1)
Given mass of rhodium(III) hydroxide = 0.730 g
Molar mass of rhodium(III) hydroxide = 120 g/mol
Putting values in equation 1, we get:
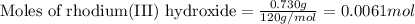
The given chemical equation follows:
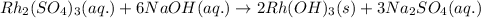
By Stoichiometry of the reaction:
When 2 moles of rhodium(III) hydroxide are produced, then 3 moles of sodium sulfate is also produced
So, when 0.0061 moles of rhodium(III) hydroxide are produced, then =
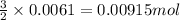 of sodium sulfate is also produced
of sodium sulfate is also produced
Now, calculating the mass of sodium sulfate from equation 1, we get:
Molar mass of sodium sulfate = 142 g/mol
Moles of sodium sulfate = 0.00915 moles
Putting values in equation 1, we get:

Hence, the theoretical yield of sodium sulfate is 1.30 grams.