Answer:
Energy
Step-by-step explanation:
The easiest way would be to remember that PV=nRT and doing fundamental dimensions on that equation:
![[nRT]=[PV]=[P][V]=ML^(-1)T^(-2)L^3=ML^2T^(-2)](https://img.qammunity.org/2020/formulas/physics/high-school/9ga3jpgwyfzml2nwsy0hkbxmgrmn02apdh.png) , so the answer is Energy.
, so the answer is Energy.
This is because we have to remember that P=F/A, and F has dimensions of mass (
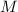 ) and acceleration (
) and acceleration (
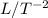 ) while A has dimensions of longitude squared (
) while A has dimensions of longitude squared (
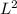 ), which gives
), which gives
![[P]=ML^(-1)T^(-2)](https://img.qammunity.org/2020/formulas/physics/high-school/yt6329ojr4njke5f1ce6r2tzdmjpjrm18u.png) . Volume has dimensions of
. Volume has dimensions of
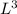 .
.
We can use any energy formula to know its dimensions, for example, from the Kinetic Energy we know that it must has dimensions of mass and velocity squared, [E]=
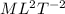 , which is what we got.
, which is what we got.